以下内容来源于 Eur Heart J
Introduction
Catheter-based renal denervation (RDN) represents an effective treatment option for patients with uncontrolled hypertension. 1–8 The US Food and Drug Administration (FDA) has recently approved the Paradise™ Ultrasound and Symplicity Spyral™ RDN systems for the treatment of uncontrolled hypertension. However, data on the safety and effectiveness of RDN in Chinese patients are lacking. This study investigated the safety and effectiveness of the basket-like Netrod™ six-electrode radiofrequency RDN system in lowering blood pressure (BP) in Chinese patients with uncontrolled primary hypertension in the presence of antihypertensive medications.
Methods
This was a prospective, multicentre, randomized, sham-controlled trial (ClinicalTrials.gov identifier: NCT03261375). Written informed consent was obtained from patients before any study procedures. Patients aged 18–65 years with uncontrolled primary hypertension [office BP ≥ 150/90 and <180/110 mmHg and mean 24 h ambulatory systolic BP (SBP) ≥ 135 mmHg] despite taking ≥2 antihypertensive drugs at a stable dose for ≥4 weeks entered a run-in period for ≥4 weeks on standardized antihypertensive therapy of nifedipine gastrointestinal therapeutic system and hydrochlorothiazide. Patients with secondary hypertension were excluded. Patients were randomized 2:1 to RDN (Netrod™ RDN System Shanghai Golden Leaf MedTech Co. Ltd, China) or sham procedure. The RDN procedure targeted all accessible renal arterial vessels (including branches and accessory renal arteries) with a diameter of 3–12 mm. All enrolled patients remained on the same doses of standard medications throughout the entire trial unless there were safety concerns. Adherence to the standard medication regimen was based on the number of tablets taken and assessed in urine using liquid chromatography with tandem mass spectrometry at the end of run-in period and the 6-month follow-up visit. Office and 24 h ambulatory BPs were assessed using the same models of sphygmomanometer for office BP and ambulatory blood pressure (ABP) according to a pre-specified protocol as per AHA guidelines. 9
The primary efficacy endpoint was the change in office SBP from baseline to 6 months post-procedure. The secondary endpoints included the changes from baseline in office diastolic BP (DBP) and 24 h ABP at 6 months post-procedure and the percentage of patients achieving target SBP (90 mmHg ≤ SBP < 140 mmHg) at 6 months post-procedure. The safety endpoint was the rate of all safety events. Both patients and study personnel assessing patients’ BP were blinded to treatment assignment until completion of the 6-month follow-up visit and subsequent unblinding.
Statistical analysis was performed using SAS® version 9.4. All statistical tests were two-sided, and a P-value of ≤.05 was considered statistically significant. BP changes were also analysed by adjusting for baseline measurements using analysis of covariance (ANCOVA). Sample size was based on the hypothesis that the BP-lowering effect of the RDN group (office SBP) was 12 mmHg greater than the sham group with standard deviation (SD) of 15 mmHg.
Results
Between November 2020 and March 2022, 205 patients from 25 centres in China with uncontrolled primary hypertension (aged 45.6 ± 8.8 years, 15.8% female) were enrolled and randomized to RDN (n = 139) or sham (n = 66).
Baseline characteristics were well balanced between groups. Mean baseline office SBP and DBP were 160.9 ± 7.4 and 99.8 ± 5.8 mmHg, respectively; mean baseline 24 h ambulatory SBP and DBP were 151.2 ± 9.4 and 96.5 ± 6.9 mmHg, respectively.
Procedure time [from the beginning of angiogram to the withdrawal of ablation catheter (prior to vessel closure)] was 84 ± 34 min in the RDN group, and total treatment time was 50.9 ± 24.1 min with a total of 16 ± 8 and 17 ± 11 ablation points in the left and right renal arteries, respectively. The ablation time of each point was 120 s with the maximal power output ≤ 9 W.
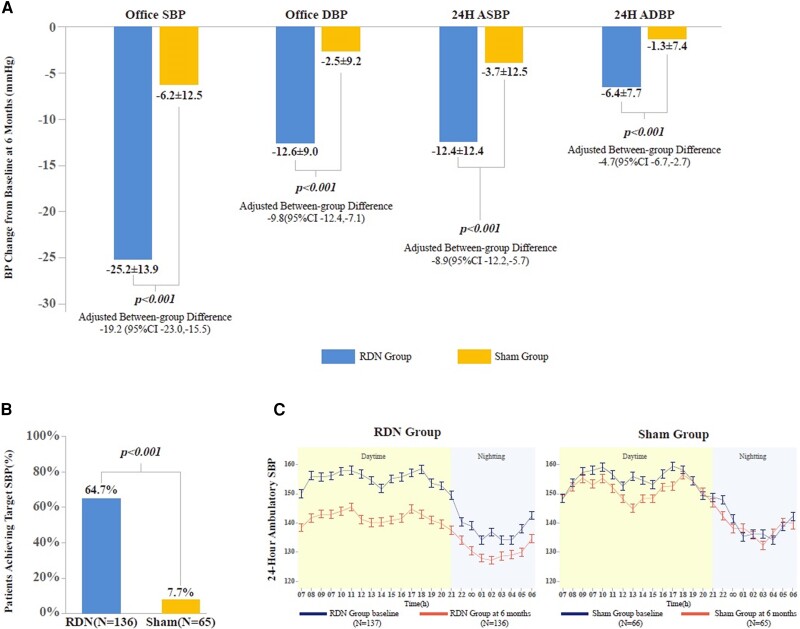
Mean (±SD) reduction in office SBP at 6 months post-procedure was 25.2 ± 13.9 mmHg and 6.2 ± 12.5 mmHg for the RDN and sham groups, respectively (between-group difference: −19.0 mmHg [95% confidence interval (CI): −23.0, −15.0]; P < .001). Reduction in office DBP from baseline to 6 months post-procedure in the RDN group was significantly greater than for the sham group (−12.6 ± 9.0 vs. −2.5 ± 9.2 mmHg [95% CI: −12.8, −7.4], P < .001). The BP reductions in 24 h ABP (both SBP and DBP) at 6 months post-procedure in the RDN group were also significantly greater (mean between-group difference: −8.7 mmHg [95%CI: −12.4, −5.0] in ASBP, −5.1 mmHg [95% CI: −7.3, −2.8] in ADBP [both P < .001]). After adjusting for each corresponding baseline BP, there were still significant differences between the RDN and sham groups across all BP outcomes (P < .001) ( Figure 1A). The percentage of patients with office SBP within target range (90 mmHg ≤ SBP < 140 mmHg) at 6 months post-procedure was significantly higher in the RDN group (64.7% vs. 7.7% P < .0001) ( Figure 1B). Hour-by-hour reductions in ambulatory SBP from baseline to 6 months post-procedure were significantly greater following RDN treatment ( Figure 1C).
Figure 1.
Blood pressure outcomes at 6 months post-procedure. (A) Changes in office and ambulatory SBP and DBP from baseline at 6 months post-procedure (RDN vs. sham control). Values are mean ± SD; RDN, renal denervation; BP, blood pressure; SBP, systolic BP; DBP, diastolic BP; 24H ASBP, 24 h ambulatory SBP; 24H ADBP, 24 h ambulatory DBP. Between-group differences were adjusted for the corresponding baseline BP measurements (using ANCOVA). Two patients (one in the RDN group and one in the sham group) were lost at 6-month follow-up. Last observation carried forward was used only for the primary endpoint (office SBP) analysis (RDN = 137, sham = 66); other endpoint analyses were based on RDN = 136, sham = 65. (B) Percentage of patients with SBP within target range (90 mmHg ≤ SBP < 140 mmHg). (C) Twenty-four hour ambulatory SBP at baseline and 6 months post-procedure (RDN vs. sham). Daytime is defined as 7 a.m. to 10 p.m., night-time 10 p.m. to 6 a.m.; error bars represent the standard error
There were no device-related serious adverse events (SAEs) reported, and the rate of procedure-related SAEs was low in both groups (.7% in the RDN group vs. 4.5% in the sham group, P = .0991). There were no reports of renal artery stenosis or deaths.
Discussion
The results demonstrated that the Netrod™ RDN System safely and significantly reduced office and ambulatory BP and facilitated BP control in a Chinese patient population with uncontrolled primary hypertension. The observed BP reductions were greater than in previously published trials, which may be attributed to the following: (i) the unique basket design of the device allowing efficient and effective renal nerve ablation with a continuous energy field ensuring circumferential targeting of all four quadrants; (ii) enrolled patients were younger compared with other trials 2–8 and had higher heart rates (≥70 b.p.m.), which is a potential predictor of response 10; (iii) the trial used a standardized regime of two antihypertensive medications to minimize drug interference; and (iv) RDN might be more effective in East Asian patients with higher salt intake and higher salt sensitivity. Further studies are required defining the effects of sodium and volume status on the response to RDN in Chinese patients—a racial difference in RDN response cannot be excluded.
Acknowledgements
Y.L. drafted the manuscript. All listed authors either participated in the study design and patient data collection or contributed to the writing of the first draft of the manuscript. Tigermed Consulting Ltd performed the statistical analysis and contributed to the writing of the study report. All authors were involved in interpretation of the data. All authors agreed on the content of the manuscript, reviewed drafts, and approved the final version. The trial was sponsored by Shanghai Golden Leaf MedTech Co. Ltd and was designed in collaboration with the steering committee and sponsor. The manuscript was written by the lead author with significant contributions from the trial's executive committee and all co-authors. The funder assisted with figure and table generation, copy editing, and formatting. We thank DRW Limited for editorial assistance, Dr Felix Mahfoud for expert review of the manuscript, all of the investigators for contributions to trial design, and Tigermed Consulting Ltd for clinical trial oversight and statistical analysis.

文章 病例29-2024:47岁男性,意识错乱、肾衰竭

测试京东医生
主任医师
北京大学医院
文章 夜间艾菲尔铁塔征

测试京东医生
主任医师
北京大学医院
文章 对一家纽约社区医院扩大C auris入院筛查方案的实用性分析

测试京东医生
主任医师
北京大学医院